Atomic Structure
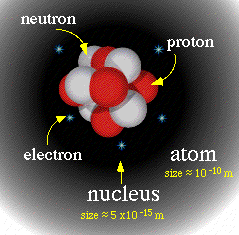
Nucleus: The central core of an atom consisting of closely packed nucleons (protons & neutrons). The nucleus comprises almost all the mass of an atom, but its radius is approximately 1/10,000th the size of the atom.
Protons: Postively-charged particles in the nucleus. The number of protons (atomic number) identifies the element and equals the number of electrons, so atoms are electrically neutral.
Neutrons: Electrically neutral particles in the nucleus. Then number of neutrons in atoms of the same element can vary (isotope).
Electrons: Particles with a negative charge and very small mass. They move around the nucleus in electron shells.
An atom can have up to 7 electron shells. From the inside out, they are called K,L,M,N,O,P, and Q shells. Each can hold up to a certain amount of electrons (shells K,L,M, and N can hold up to 2, 8, 18, and 32 electrons respectively). The further away the shell is from the nucleus, the higher the energy of its electrons. If the outer shell is full or has an octet (8 electrons), the atom is very stable.
Mass Number (A): The number of protons & neutrons in a nucleus. It is the whole number nearest to the relative atomic mass of the atoms, and is important in identifying isotopes.
Atomic Number (Z): The number of protons in a nucleus (also the number of electrons around it). All the atoms with the same atomic number are of the same element.
Neutron Number (N): The number of neutrons in a nucleus, calculated by subtracting the atomic number from the mass number. A-Z=N
What are the 10 most abundant elements?
10) Sulfur, 9) Magnesium, 8) Iron, 7) Silicon, 6) Nitrogen, 5) Neon, 4) Carbon, 3) Oxygen, 2) Helium, 1) Hydrogen
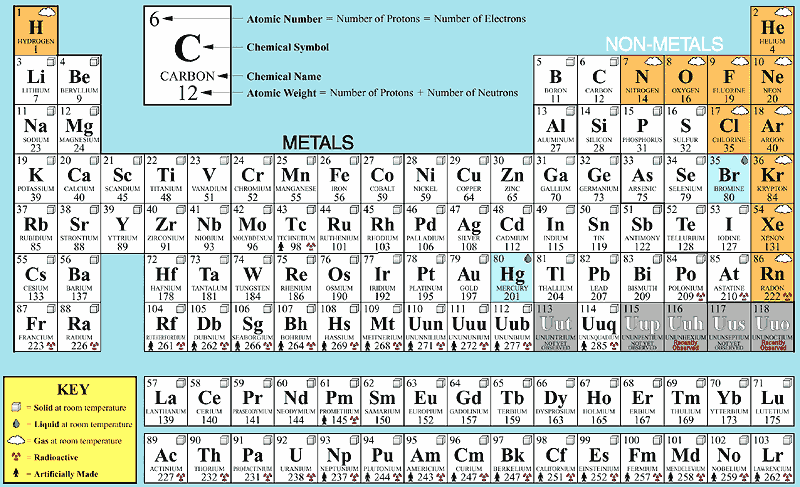
There are currently 118 known elements.
87 are metals
12 are gasses
2 occur as liquids (Bromine & Mercury)
26 are radioactive
24 are made only in particle accelerators
In addition, each element can have a number of isotopes. The two newest
members of the table of the elements, with atomic numbers of 113 and
115, were recently made in accelerators in Russia.